Acids on your skin? Sounds scary? It does, but they can do a lot for you. To see how, let's start with the top layer of your skin, the epidermis.
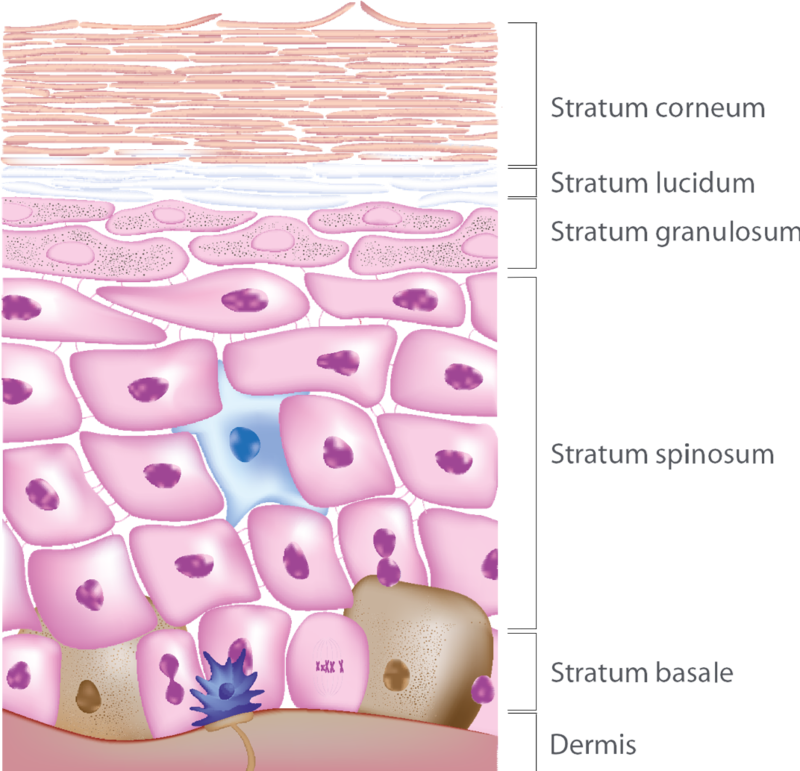 The epidermis doesn't have any blood supply - any nourishment it gets is from the layer below it, the dermis. New cells form in the lowest level of the epidermis, the stratum basale, or basal layer. These new skin cells push the other cells upwards. As the cells move up, they grow larger, dehydrate and become harder until they eventually end up as hard, dead cells in the stratum corneum (horny layer). Most of your skin has only four layers; the stratum lucidum is found only on the bottom of your feet and your palms. The topmost cells fall off, and the entire upper layer (the stratum corneum) gets replaced every two weeks. So the whole epidermis gets replaced about every four weeks.
The epidermis doesn't have any blood supply - any nourishment it gets is from the layer below it, the dermis. New cells form in the lowest level of the epidermis, the stratum basale, or basal layer. These new skin cells push the other cells upwards. As the cells move up, they grow larger, dehydrate and become harder until they eventually end up as hard, dead cells in the stratum corneum (horny layer). Most of your skin has only four layers; the stratum lucidum is found only on the bottom of your feet and your palms. The topmost cells fall off, and the entire upper layer (the stratum corneum) gets replaced every two weeks. So the whole epidermis gets replaced about every four weeks.
This replacement process can be slowed by aging, hormones, diet changes, and weather. Dead cells that hang around too long can make your skin look duller, your pores look bigger, and contribute to acne. That's why it's a good idea to exfoliate.
There are two ways to exfoliate: physical and chemical. Physical exfoliation is scrubbing with rough cloths, sponges, physical devices, brushes, or scrubs. Chemical exfoliators are primarily acids.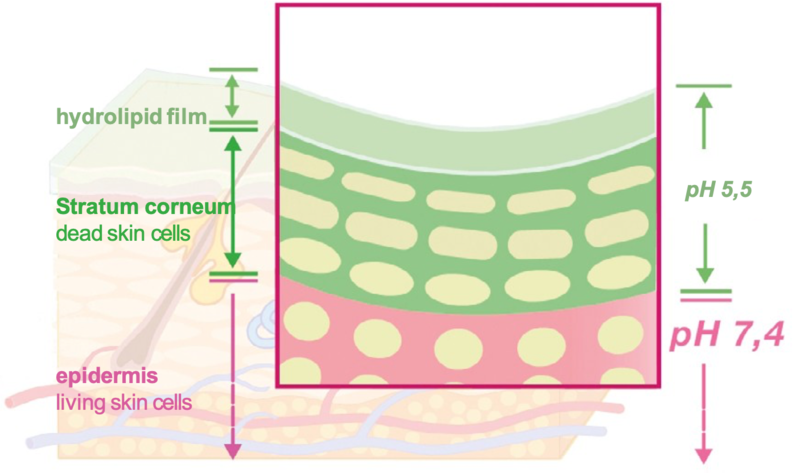 We aren't entirely sure how these chemical exfoliators work, but we know they do. While physical exfoliators work well on most of your body, the skin on your face is much more delicate. Scrubbing can irritate or damage this fragile skin. Your skin also has an acid barrier (called the "acid mantle) that protects it. As you can see in the picture, the pH of the skin and mantle are around 5.5 (more on pH in a moment).
We aren't entirely sure how these chemical exfoliators work, but we know they do. While physical exfoliators work well on most of your body, the skin on your face is much more delicate. Scrubbing can irritate or damage this fragile skin. Your skin also has an acid barrier (called the "acid mantle) that protects it. As you can see in the picture, the pH of the skin and mantle are around 5.5 (more on pH in a moment).
Physical exfoliation disrupts this acid mantle, but acids do not. Most acids also have additional benefits. Some acid molecules are small enough to reach into the deeper layers of your skin. That allows them to improve skin thickness, encourage faster cell turnover, and even encourage new collagen generation, which helps fine lines and wrinkles. Acids can also even out your skin tone and remove some discolorations. If used with care, the multiple benefits they provide would make them worth using, even if they did nothing for exfoliation!
But which products to choose, which acids to use? There are so many out there! The skincare industry is no help. There are so many ingredients and claims about products that it is difficult to make the right decision. And the prices are unbearably high even though the core ingredients that actually do the work are inexpensive! Brands price products that include them at a premium because they work so well. And, of course, greed pulls the rest of them in once one brand does it. Just look; you'll see the same ingredients in all products. But to justify the outrageous prices and suck consumers in, they add fancy-sounding ingredients with little or no evidence. The actual concentrations of the truly effective elements are usually hard to find and sometimes surprisingly low. As a consumer, this is super annoying. And discouraging. After all, who wants to spend $150 only to find that it has done nothing for you?
The Solution: The Ordinary
A recent brand, "The Ordinary" (parent company Deciem), has solved most of these issues. Their product line is excellent. They base their formulations on science, list the bottles' concentrations, and the prices are extraordinarily reasonable. I am using two of the products, which last about six weeks. The total cost for both is under $14! These are extraordinary prices!
I also appreciate their marketing, which is informative and refreshingly free of hype. The problem with informative, hype-free marketing is that consumers need to know more about the products. Conventional wisdom argues that beauty consumers are too dumb and lazy to inform themselves. But The Ordinary has shown this to be false. Consumers have responded to The Ordinary by buying from them in droves and have made The Ordinary a major player in a remarkably short time. There is a market for intelligent beauty consumers!
What follows are the basics about acids that I've managed to learn as I tried to become an informed consumer of beauty products. Later articles will discuss specific acids.
Acid Basics
Acids are molecules that release hydrogen atoms when you dissolve them. The stronger the acid, the more easily it lets go of its hydrogen atoms when placed in a solution. These single hydrogen atoms are called hydrogen ions. The more hydrogen ions are floating around in a solution, the more acidic it is. The pH values in product literature tell you how acidic the solution is. To help you get a feeling for what pH values mean, here's a pH table that also lists some everyday household products:
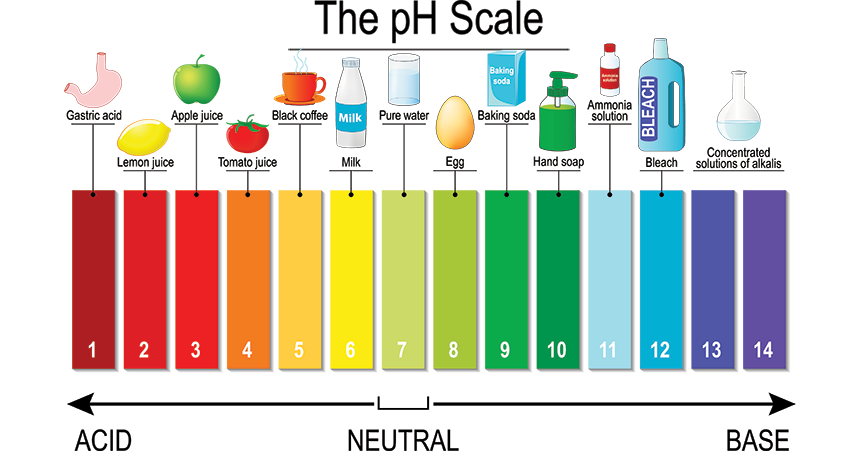
Perhaps surprisingly, the lower the pH, the more acid the solution! The most acidic solutions have a pH of one, growing progressively less acid until you reach the neutral pH of seven. Every step on the pH scale changes the strength of the acid by a factor of 10, so apple juice (pH 3) is 10-times more acidic than tomato juice (pH 4). Distilled water is completely neutral with a pH of 7. Above that, we have the anti-acids, acid opposites, or "bases." These are the chemical opposites of acids. If you mix acids and bases, they will react and neutralize each other. Try mixing vinegar and baking soda; you will see what I mean. (If you have children, they love that activity.)
As we mentioned earlier, the skin and acid mantle have a pH which is (ideally) around 5.5. You might have noticed in the pH table that soap's pH puts it in the anti-acid zone of the table! It's fairly high, around 10. So when you use soap to wash your face, it tends to neutralize the skin's acidity, leaving it closer to a neutral pH of 7. The problem with that is that bacteria love a neutral pH. Those little guys flourish there. As you probably know, bacteria can contribute to many skin problems, so this is another reason I like acid for exfoliating and cleansing the skin.
Another number you will see in product literature is an acid's pKa value. That's a measure of how strong the acid is. Remember that the strong acids give up their hydrogen atoms easily when dissolved? So pKa is also a measure of how soluble the acid is when you dissolve it. If you place hydrochloric acid, a powerful acid with a pKa of -6.3, into a solution, it will dissolve entirely and release all hydrogen atoms. But the acids we put on our skin have a pKa between 3 and 4, so only some molecules will give up their hydrogen atoms. Others won't - they remain complete and undissolved.
Undissolved atoms are electrically neutral (they are called "free acid"). If they dissolve and give up a hydrogen ion (which is positive), then they have a negative charge. The charge matters because the skin only allows neutral atoms to pass through it, so the neutral molecules (the free acid) are the biologically active ones. We say they are "bioavailable." That's where pKa becomes important: we want plenty of free acid molecules, and pKa can tell us how much acid we need to do that.
An exciting fact: when the pH of a solution is equal to the pKa value of the acid, then 50% of the acid is free, and 50% is dissolved. Let's say you have an acid with a pKa of 3. Depending on the pH, you will have the following situations:
| pH | Free Acid | Ionized Acid |
|---|---|---|
| 4 | 10% | 90% |
| 3 | 50% | 50% |
| 2 | 90% | 10% |
| 1 | 99% | 1% |
Suppose you buy a bottle of pKa 3 acid from a company at a pH of 3. The bottle says the concentration of the acid is 8%. Since the pKa and pH values are equal, you instantly know that half (4%) of that acid is biologically active (free acid) and 4% is not. That's a pretty good situation since you know that at least 1/2 of the active ingredients will go where you want them.
To drive home the relationship between pH and bioavailability, here are the results from a real-world study that I found that compared the absorption of two of the most commonly used skin acids at different pHs:
| Acid | pKa | pH | Absorption after 24 hours |
|---|---|---|---|
| Glycolic Acid | 3.83 | 3 | 27.0% |
| 7 | 3.5% | ||
| Lactic Acid | 3.86 | 3 | 30.0% |
| 7 | 10.0% |
Look at the huge difference in absorption having a low pH makes! All of this does matter.
(Here's the study referenced: (Kraeling MK & Bronaugh RL, In vitro percutaneous absorption of alpha-hydroxy acids in human skin, J Soc Cosmet Chem 1997, 48, 187.)
Since we get more bioavailable acid at a lower pH, why not make all products at a pH of zero? Well, because that would burn your face off! Low pH's do burn our faces off to varying degrees anyway. You'll know from the stinging, redness, itchiness, and peeling whether or not your skin can handle it. (I wrote about this a bit in Part 11, where I talked about your skin threshold.)
What happens to the other 4% of our ionized acid? Does it just sit there on top of the skin? No, it doesn't. As the free acid gets absorbed into the skin, the percentage of free acid on the skin's surface drops. As that happens, some ionized acids reconnect with their hydrogen atoms and become free acids. So, in theory, given enough time, all the acid on your skin could become bioavailable. Some companies use this to market acid products at higher pHs as "time-release" or "buffered ."Since there is less free acid, there is also less skin irritation. But if the pH is too high, the acid will be absorbed too slowly, and it will be too diluted on your skin. It's like the difference between drinking an entire bottle of vodka vs. dumping the bottle into a swimming pool and drinking all the water in the pool. Only one of those activities will get you drunk.
The point of the swimming pool analogy is that concentration matters. An acid with a pKa of 3 at 10% concentration with a pH of zero WILL burn your face off. But a 50% concentration with a pH of 4 will burn it just as badly.
The Take Home Message
Acids are good.
The amount of acid your skin will absorb is determined by the pH. Lower pHs increase the amount of acid the skin absorbs and causes increased irritation (with no time-release properties).
pH's higher than the recommended range might work, but not as well. They might work so poorly that you won't see any effect. Increasing the concentration of the acid can help it operate at a higher pH but will also increase the potential for irritation.
That's it for now. Part 3 introduces some great and useful skincare acids! If you have comments or questions, please send the nice ones to me!